Hydrate-Based CO₂ Capture
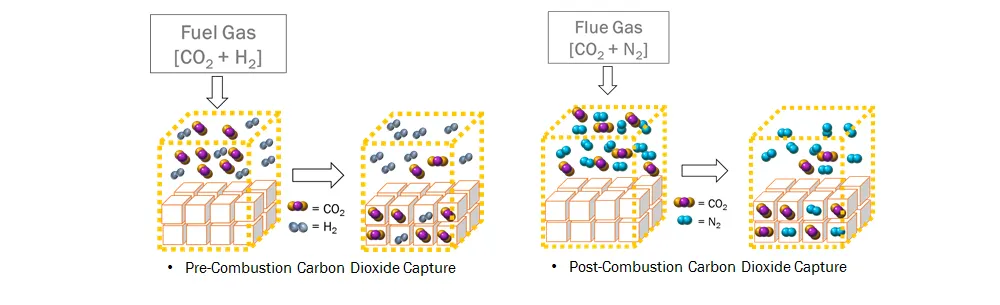
The concept of a hydrate-based CO₂ capture is based on selective partitioning of CO₂ from CO₂ + H₂ gas mixtures (pre-combustion) or CO₂ + N₂ gas mixtures (post-combustion) during hydrate formation. CO₂ has a higher selectivity in the hydrate phase, since CO₂ is more thermodynamically stable than H₂ or N₂. A main drawback of the hydrate-based CO₂ capture process is that it requires high pressure for gas hydrate formation. Adding a small amount of organic compounds called thermodynamic promoters such as tetrahydrofuran (THF) and cyclopentane (CP) can significantly reduce the hydrate equilibrium pressure at a given temperature. Semiclathrates formed by quaternary ammonium salts such as tetra-n-butyl ammonium bromide (TBAB), chloride (TBAC), and fluoride (TBAF) have been considered as a new potential CO₂ capture material due to the presence of empty cages that can capture small-sized gas molecules at low pressure and high temperature conditions.
An Innovative CO₂ Storage Method
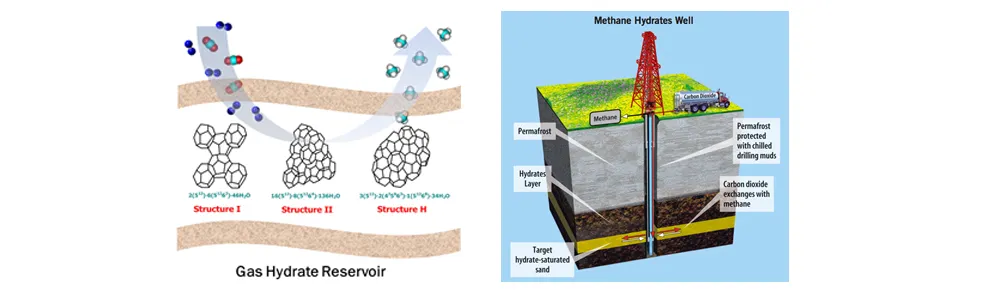
Naturally occurring gas hydrates, primarily comprised of methane (CH₄), have recently emerged as a promising future energy source because they are found abundantly in the deep ocean sediments of continental margins and permafrost regions. The amount of natural gas stored in the known gas hydrate reservoirs has been reported to exceed the energy contained in the total fossil fuel reserves by at least twofold. Moreover, the sequestration of CO₂ into these natural gas hydrate reservoirs is attracting widespread interest in the field of CO₂ capture and storage (CCS).
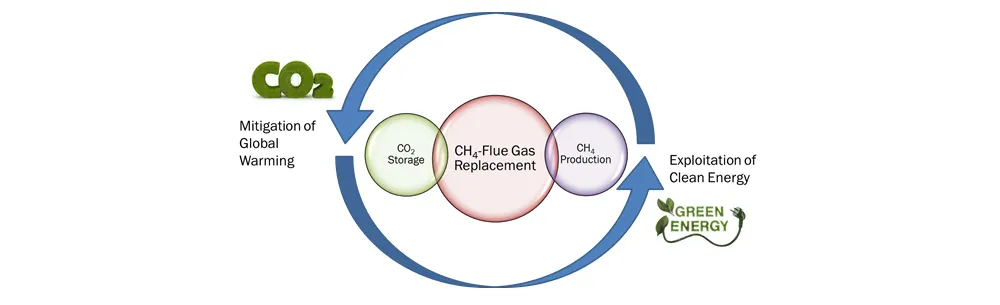
Given that global warming has primarily been attributed to anthropogenic CO₂ emissions, the replacement of CH₄ with CO₂ in the natural gas hydrate reservoirs would function as both effective CH₄ recovery for securing future clean energy and innovative CO₂ storage for mitigating global warming, and also reduce the geo-mechanical hazards that could occur when using conventional production methods such as depressurization and thermal stimulation.
Recently, the injection of flue gas instead of pure CO₂ into natural gas hydrate reservoirs has been proposed for both CO₂ sequestration and CH₄ exploitation. Because the CH₄-flue gas replacement enables not only the effective exploitation of CH₄, but also exclusion of the CO₂ separation step, it could be a more attractive option for both global warming gas storage and energy recovery.