Carbon Dioxide Capture Using Solid Sorbents
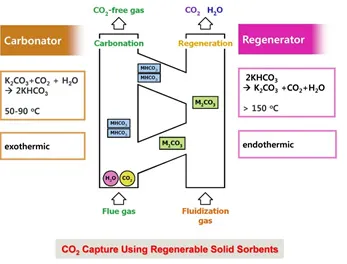
Recently CO₂ capture using regenerable solid sorbents has been studied as an innovative concept for CO₂ recovery. CO₂ is efficiently removed from the flue gas stream by reaction with solid sorbents while regeneration produces an off-gas containing only CO₂ and H₂O. The condensation of an off-gas generates highly pure CO₂, which is suitable for chemical feed stock or sequestration. Because solid sorbents are made of cheap alkali metals and carbonated sorbents can be regenerated with heat only from the flue gas stream, the solid sorbent process for CO₂ capture is thought to be cost-effective and energy-efficient.
Thermal Degradation of Amine Solutions for Carbon Dioxide Capture
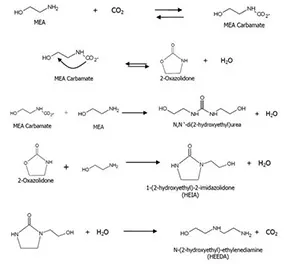
There are several ways to capture CO₂ from the flue gas stream, but the most well-known one is using amine, such as monoethanolamine (MEA). Amines are organic bases, which have the ability to react with weak acids such as CO₂ at ambient temperatures to form amine carbamates. This process is reversed by applying heat in the stripper. However, at elevated temperature and CO₂ concentration, amines can go through an irreversible degradation process termed carbamate polymerization which causes a loss of acid gas absorption capacity, increased solution viscosity, increased corrosion and potential process upsets such as foaming. Therefore, quantifying and analyzing the compounds which are the results of amine thermal degradation are very important for the stable and long-term operation of CO₂ absorption processes.